Technology R&D



Human pluripotent stem cells (PSC: iPS and ES)-based
pancreatic cells for diabetes treatment
Development of new drugs to treat metabolic
dysfunction-associated steatohepatitis (MASH)
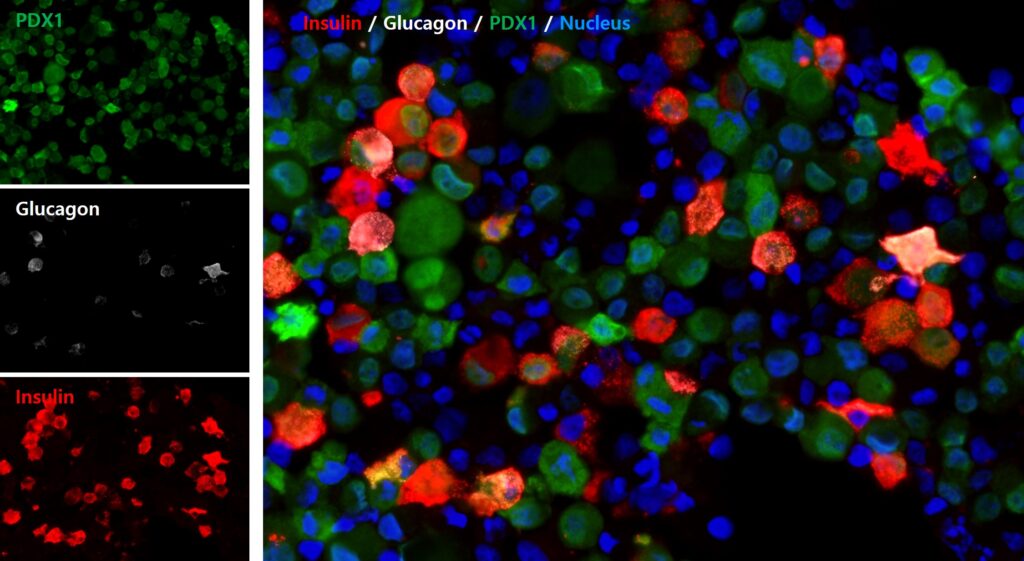
Diabetes is a chronic disease that affects about 9% of the world's population, and insulin injection is essential, especially for type 1 diabetes patients. Our technology aims to cure the disease by differentiating human pluripotent stem cell into pancreatic cells. If autologous induced pluripotent stem cells are used, immunosuppressants are not required. Moreover, the surgical procedure can be easily adjusted according to the patient's convenience, because this technology can produce the pancreatic cells in vitro as previously scheduled. Through animal studies, we have confirmed these cells' ability to control blood sugar, and we will accelerate the differentiation process further to ultimately produce stem cell-derived pancreatic cells.
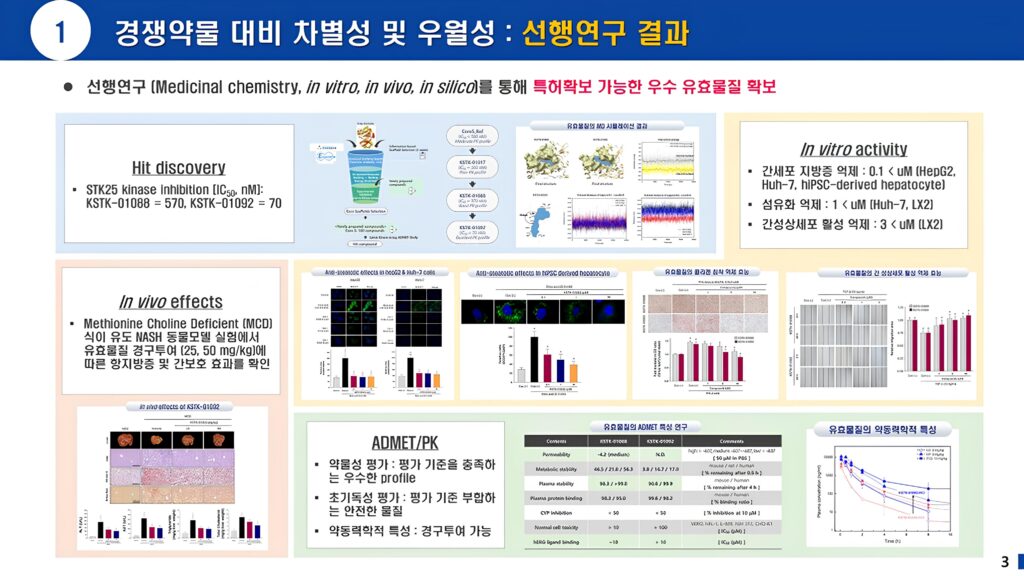
Metabolic dysfunction-associated steatohepatitis (MASH) is a serious disease that can lead to cirrhosis, liver failure, and liver cancer, and to date, Madrigal's Rezdiffra, which recently received FDA approval, is the only treatment. STK25 has been recognized as a potential new target for MASH therapeutic drugs because when it is overactivated, it leads to the promotion of insulin resistance, homeostasis imbalance of glucose, and induction of liver fat accumulation. Increased STK25 mRNA and protein have been identified in the liver of MASH patients, and STK25 inhibition has been reported to alleviate MASH symptoms. In addition, several recent studies have suggested that STK25 blockade is effective in treating MASH and related metabolic diseases. Based on this, we are developing therapeutic new drug, STK chemical inhibitor for MASH.
Diabetes is a chronic disease that affects about 9% of the world's population, and insulin injection is essential, especially for type 1 diabetes patients. Our technology aims to cure the disease by differentiating human pluripotent stem cell into pancreatic cells. If autologous induced pluripotent stem cells are used, immunosuppressants are not required. Moreover, the surgical procedure can be easily adjusted according to the patient's convenience, because this technology can produce the pancreatic cells in vitro as previously scheduled. Through animal studies, we have confirmed these cells' ability to control blood sugar, and we will accelerate the differentiation process further to ultimately produce stem cell-derived pancreatic cells.

Development of new drugs to treat metabolic
dysfunction-associated steatohepatitis (MASH)

Metabolic dysfunction-associated steatohepatitis (MASH) is a serious disease that can lead to cirrhosis, liver failure, and liver cancer, and to date, Madrigal's Rezdiffra, which recently received FDA approval, is the only treatment. STK25 has been recognized as a potential new target for MASH therapeutic drugs because when it is overactivated, it leads to the promotion of insulin resistance, homeostasis imbalance of glucose, and induction of liver fat accumulation. Increased STK25 mRNA and protein have been identified in the liver of MASH patients, and STK25 inhibition has been reported to alleviate MASH symptoms. In addition, several recent studies have suggested that STK25 blockade is effective in treating MASH and related metabolic diseases. Based on this, we are developing therapeutic new drug, STK chemical inhibitor for MASH.

